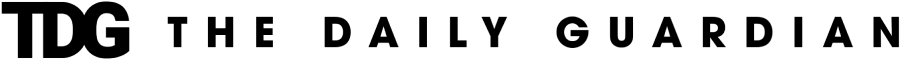One in four people experience mild, short-lived systemic side effects after receiving either the Pfizer or AstraZeneca vaccine, with headache, fatigue and tenderness the most common symptoms, suggest the findings of a new study.
Most side effects peaked within the first 24 hours following vaccination and usually lasted 1-2 days.
The study published today in The Lancet Infectious Diseases is the first large scale study to compare the two vaccines and investigate the prevalence of mild side effects of the UK’s vaccination programme.
The analysis by researchers from King’s College London of data from the ZOE COVID Symptom Study app reassuringly found much fewer side effects in the general population with both the Pfizer and AstraZeneca vaccines than reported in trials. The study also reports a significant decrease of infection rates from 12 to 21 days after the first dose of the Pfizer (58% reduction) and AstraZeneca (39% reduction) vaccines compared to a control group.
The drop in infection at least 21 days after the first dose for Pfizer is 69% and for AstraZeneca 60%. This large-scale analysis examined the differences of reported side effects from the two vaccines currently distributed in the UK. Systemic effects – meaning side effects excluding where the injection took place – included headache, fatigue, chills and shiver, diarrhoea, fever, arthralgia, myalgia, and nausea; whilst local side effects – meaning side effects where the injection took place in the arm – included pain at the site of injection, swelling, tenderness, redness, itch, warmth and swollen armpit glands.
The data comes from 627,383 users of the ZOE COVID Symptom Study app who self-reported systemic and local effects within eight days of receiving one or two doses of the Pfizer vaccine or one dose of the AstraZeneca vaccine between December 8 and March 10.
Summary of findings: -25.4 per cent of vaccinated people indicated suffering from one or more systemic (excluding the area where the injection took place) side effects, whereas 66.2 per cent reported one or more local (at the injection site) side effects. -13.5 per cent of participants reported side effects after their first Pfizer dose, 22.0 per cent after the second Pfizer dose and 33.7 per cent after the first AstraZeneca dose.
The most reported systemic side effect was a headache. 7.8 per cent of people reported suffering from headaches after the first Pfizer dose and 13.2 per cent after the second Pfizer dose. 22.8 per cent of people who had the first dose of the AstraZeneca vaccine reported a headache. The second most reported systemic side effect was fatigue. 8.4% and 14.4% of participants reported fatigue after first and second dose of Pfizer vaccine and 21.1% reported fatigue after their first dose of AstraZeneca vaccine. The most common local side effect was tenderness: 57.2 per cent and 50.9 per cent after the first and second dose of Pfizer vaccine, and 49.3% after the first dose of AstraZeneca vaccine.
Importantly, this research identifies that side effects were more common among people under 55 years of age and among women.
Also, participants who had a confirmed case of prior COVID-19 were three times more likely to have side effects that affect the whole body after receiving doses of the Pfizer vaccine than those without known infection and almost twice more likely after the first dose of the AstraZeneca vaccine.
People with prior known COVID-19 infection were also more likely to experience local effects. In Phase III clinical trials of the Pfizer vaccine, the most common side effects were pain at the injection site (71-83 per cent), fatigue (34-47 per cent) and headache (25-42 per cent), however, the real-world analysis found less than 30 per cent of users complained of injection site pain and less than 10 per cent of fatigue and headache after the first dose.
Similarly, in Phase III trials for the AstraZeneca vaccine, systemic side effects were found in 88 per cent of younger participants (18-55 years) after the first dose but this study found a significantly lower rate of 46.2 per cent after the first dose.
While rates of side effects were much lower than expected from clinical trials, rates of infection after vaccine were reassuring after two three weeks and in line with findings from previous trials and recent data from the Israeli vaccination programme. Professor Tim Spector OBE, lead scientist on the ZOE COVID Symptom Study app and Professor of Genetic Epidemiology at King’s College London said: “The data should reassure many people that in the real world, after-effects of the vaccine are usually mild and short-lived, especially in the over 50’s who are most at risk of the infection. Rates of the new disease are at a new low in the UK according to the ZOE app, due to a combination of social measures and vaccination and we need to continue this successful strategy to cover the remaining population”.
“The results also show up to 7 per cent protection after 3 weeks following a single dose, which is fantastic news for the country, especially as more people have now had their second jabs.” Dr Cristina Menni, first author of the study from King’s College London said: “Our results support the aftereffects safety of both vaccines with fewer side effects in the general population than reported in the Pfizer and AstraZeneca experimental trials and should help allay safety concerns of people willing to get vaccinated.”