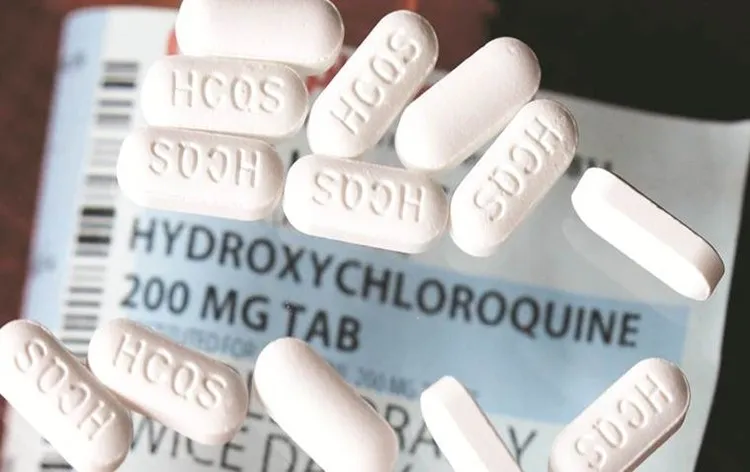
Study That Promoted Trump’s ‘Miracle Drug’ as COVID Treatment Retracted: The Controversy Behind Hydroxychloroquine
In the early days of the COVID-19 pandemic, President Donald Trump championed the use of hydroxychloroquine, an anti-malarial drug, as a “miracle” treatment for the virus. This endorsement was made despite a lack of solid scientific evidence supporting its efficacy against COVID-19. The drug was touted by the president and other political figures as a potential game-changer, capable of preventing or treating the virus. However, the claim was met with skepticism by many health experts and researchers, who cautioned against its use without proper clinical trials.
One of the studies that helped fuel the promotion of hydroxychloroquine as a COVID-19 treatment was later retracted. The study, which was published in The Lancet, one of the most prestigious medical journals in the world, was based on data from a company called Surgisphere. The study, titled “Hydroxychloroquine or Chloroquine with or without a Macrolide for Treatment of Covid-19,” claimed to provide evidence that hydroxychloroquine was ineffective in treating COVID-19 and could even increase the risk of death in patients. However, the study was eventually retracted after concerns over the authenticity of the data and the integrity of the research methods used.
The retraction of this study raised important questions about the role of scientific research in shaping public health decisions, especially during a global health crisis. It also highlighted the dangers of political influence on medical science and the need for rigorous scientific validation before drugs are recommended for widespread use. This article will examine the study that promoted hydroxychloroquine as a potential COVID-19 treatment, the controversy surrounding its findings, and the eventual retraction of the paper.
The Rise of Hydroxychloroquine in the COVID-19 Pandemic
When COVID-19 first emerged in late 2019 and early 2020, the global medical community scrambled to find effective treatments. As the virus spread rapidly, causing millions of infections and deaths worldwide, there was an urgent need for a solution. In March 2020, President Trump began publicly promoting hydroxychloroquine as a possible treatment for COVID-19, despite the lack of clinical evidence to support its use.
Hydroxychloroquine is an anti-malarial drug that has been used for decades to treat malaria, lupus, and rheumatoid arthritis. It has a history of being relatively safe when used under medical supervision, but its effectiveness against COVID-19 was unproven. Trump’s endorsement of the drug came at a time when the world was looking for any potential remedy to the pandemic. The president made bold claims about the drug, calling it a “game-changer” and a “miracle” solution for COVID-19.
At the time, many people, particularly in the U.S., turned to hydroxychloroquine in hopes that it would prevent or treat the virus. This led to shortages of the drug, with some people even seeking it out without a prescription. Meanwhile, health experts, including the U.S. Food and Drug Administration (FDA) and the World Health Organization (WHO), warned against using hydroxychloroquine outside of clinical trials, citing the lack of evidence supporting its effectiveness.
In the midst of this confusion, a large-scale study was published in The Lancet in May 2020, which aimed to examine the effects of hydroxychloroquine on COVID-19 patients.
The Lancet Study and Its Promising Findings
The study published in The Lancet was designed to assess the impact of hydroxychloroquine and chloroquine on patients with COVID-19. The paper claimed to have analyzed data from over 96,000 patients in 671 hospitals across six continents. According to the study, hydroxychloroquine was not only ineffective in treating COVID-19, but it also increased the risk of serious heart-related side effects and death in patients. This was a significant blow to the narrative that hydroxychloroquine could be a potential treatment for COVID-19, especially after the endorsement from political figures like Trump.
The study concluded that hydroxychloroquine and chloroquine did not provide any benefit to patients with COVID-19 and were associated with increased mortality rates. The findings were widely publicized, and many news outlets and health authorities cited the study as evidence that hydroxychloroquine should not be used as a treatment for COVID-19.
The publication of this study further fueled the debate over the drug’s efficacy and raised concerns about the safety of hydroxychloroquine, especially in a time when many were turning to it as a potential cure. The study’s impact was significant, and it added to the growing body of evidence that suggested the drug was not a viable solution for treating COVID-19.
The Scrutiny of the Data and Methodology
Despite the study’s widespread attention and its influence on the public perception of hydroxychloroquine, cracks soon began to appear in the research. Experts and researchers started questioning the methodology used in the study and the authenticity of the data. The study had been based on data provided by a company called Surgisphere, which specialized in aggregating and selling medical data. The company claimed to have access to a vast database of patient records from hospitals around the world.
However, concerns were raised about the quality and accuracy of the data provided by Surgisphere. Some of the data appeared to be inconsistent or lacked transparency, raising doubts about the validity of the findings. Several prominent scientists and researchers, including experts in the field of epidemiology, expressed concerns that the study may have been based on flawed or fabricated data.
As a result of these concerns, the authors of the study conducted an internal review of the data, and the publication was eventually retracted in June 2020. The Lancet issued a statement acknowledging the issues with the data and the need for further investigation into the methodology. The retraction of the study sent shockwaves through the scientific and medical communities, as it had been widely cited in discussions about the use of hydroxychloroquine for COVID-19 treatment.
The Role of Political Influence in Medical Science
The controversy surrounding the study and its retraction highlighted the dangers of political influence on scientific research and public health policy. The endorsement of hydroxychloroquine by political leaders like Trump had created an environment in which scientific evidence was often overshadowed by political rhetoric. The promotion of the drug as a “miracle cure” for COVID-19, without sufficient evidence to back up these claims, led to widespread misinformation and confusion among the public.
In many ways, the hydroxychloroquine saga illustrated the tension between science and politics in times of crisis. While the scientific community urged caution and recommended evidence-based approaches to treating COVID-19, political leaders often prioritized expediency and public sentiment over rigorous scientific inquiry. The rush to promote a quick fix, such as hydroxychloroquine, was driven by a desire for immediate solutions to the global pandemic, but it also came with serious risks, including the potential for harm to patients who took the drug without proper medical supervision.
The retraction of the Lancet study further reinforced the importance of relying on solid scientific evidence when making public health decisions. The role of peer-reviewed research, independent verification of data, and adherence to rigorous scientific methods is essential to ensuring the safety and efficacy of medical treatments.
The Aftermath of the Retraction: Lessons Learned
In the aftermath of the retraction of the Lancet study, there were several key takeaways for both the scientific community and the general public. First and foremost, the episode underscored the importance of transparency and integrity in scientific research. Data manipulation or errors in methodology can undermine the credibility of entire studies and erode public trust in medical science.
Second, the incident highlighted the dangers of misinformation and the consequences of making health recommendations without sufficient evidence. The promotion of hydroxychloroquine as a miracle treatment without robust scientific backing caused confusion and led to unnecessary risks for individuals who took the drug based on political endorsements rather than medical advice.
Lastly, the controversy over hydroxychloroquine demonstrated the need for more rigorous and transparent clinical trials, particularly during global health crises. In the rush to find solutions to COVID-19, some treatments were touted prematurely, without the proper testing required to confirm their safety and efficacy. Going forward, it is essential that all proposed treatments undergo thorough testing and validation before they are recommended for widespread use.
The Ongoing Battle for Evidence-Based Medicine
The story of hydroxychloroquine’s promotion as a COVID-19 treatment is a cautionary tale about the dangers of political interference in public health. The retraction of the Lancet study, which was once seen as a cornerstone of the argument against hydroxychloroquine, highlighted the need for scientific rigor and integrity in medical research.
As the world continues to fight the COVID-19 pandemic, it is more important than ever to rely on evidence-based medicine and to ensure that medical decisions are based on the best available science, not political agendas or unverified claims. The hydroxychloroquine controversy serves as a reminder that, in matters of public health, the truth must always come first.