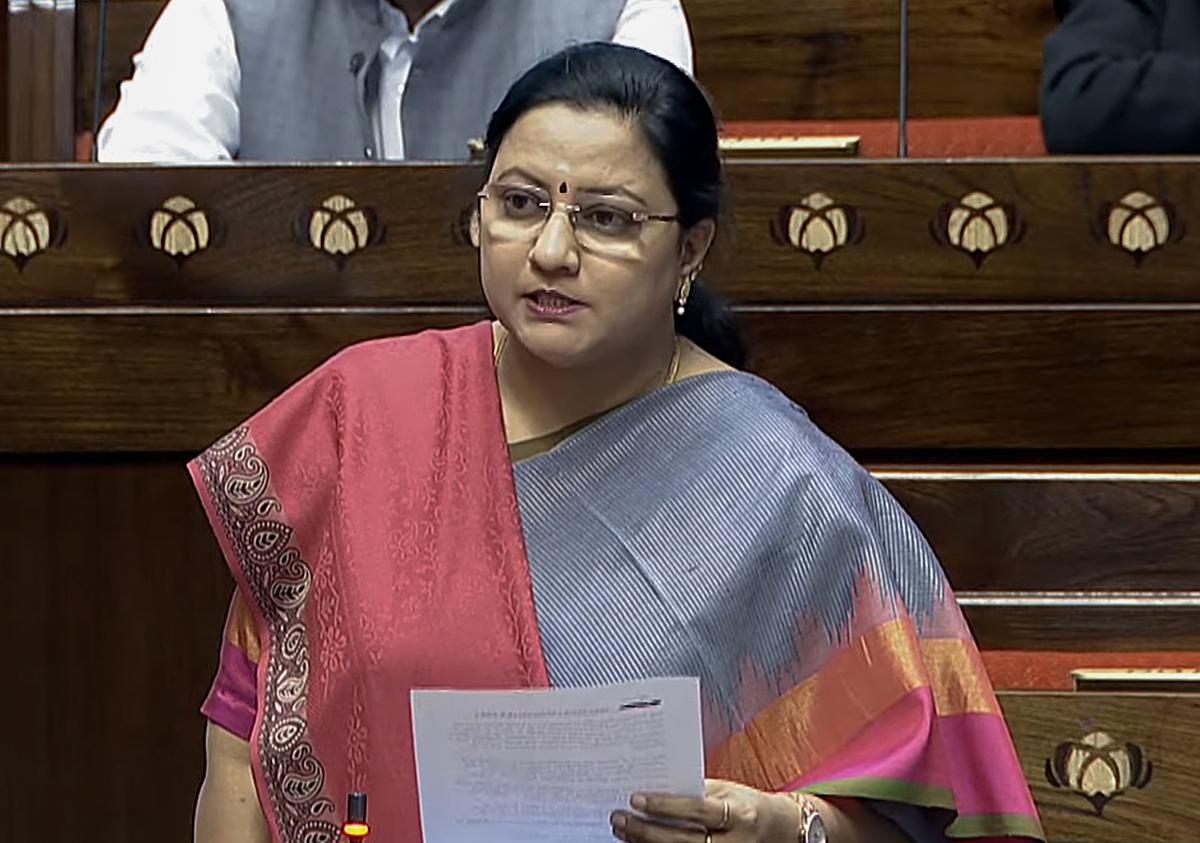
DMK Rajya Sabha member Dr. Kanimozhi NVN on Monday raised the concern over rising cases of dubious drug trials having serious repercussions on the healthcare sector in the country.
While speaking in the Rajya Sabha, the DMK leader said, “I want to bring notice to house about a very serious raised by the ethical committee of the National Institute of Virology, Pune about the rising cases of dubious drug trials in India which have serious repercussions on the entire healthcare sectors.”
She said that it has also raised a serious question about the monitoring and quality control of the clinical studies in our country.
The DMK leader further pointed out that recently, the Drug Controller General of India (DCGI) has received an appeal to investigate the critical gaps in clinical trials by a pharma company to develop a biosimilar treatment of breast cancer.
“For a company to conduct trials, all trials have to be registered with the central trial registry of India,” she said, adding that the most important factor in such trials is the “genuineness” of the original product which is considered the goal standard.
“Any doubt about the authenticity of the gold standard is bound to question the integrity of the whole trial and impacts the effectiveness of the biosimilar copy being tested,” she highlighted.
She also said that such allegation are that the trials are being conducted just 4 months before the procurement of the gold standard drug from the original manufacturer.
“As a principal, the national accreditation board if hospitals and the quality control of India had called the ethics committee of the medical colleges and the big hospitals as dysfunctional as they adjusts namesake and do not scrutinize properly in many instances,” she said.
She also said that if this penurious thing going undetected by the IECs and the IRBs of the frothy premiere institutes of the country illustrates the porosity of the checks and balances of the ethical conduct of clinical trial.
“This raises a serious question about the authenticity of the trial data by no slight of hand can anomaly be brushed under a carpet. The human lives are at the stake if this drug cease the light of the dubious trials.
“Unfortunately the same pharma company has a substantial large contribution through electoral bonds. The data released by the Election Commission on March 14 has revealed 35 pharma companies in India have contributed nearly thousand crores of to political parties across the state and center through the companies which have been invested for poor quality drug which they are purchased through the bonds,” she said.
Kanimozhi NVK said that as the member of the medical fraternity “I caution the Union government to take stringent action against all those involved in this unethical practices in clinical trials”.