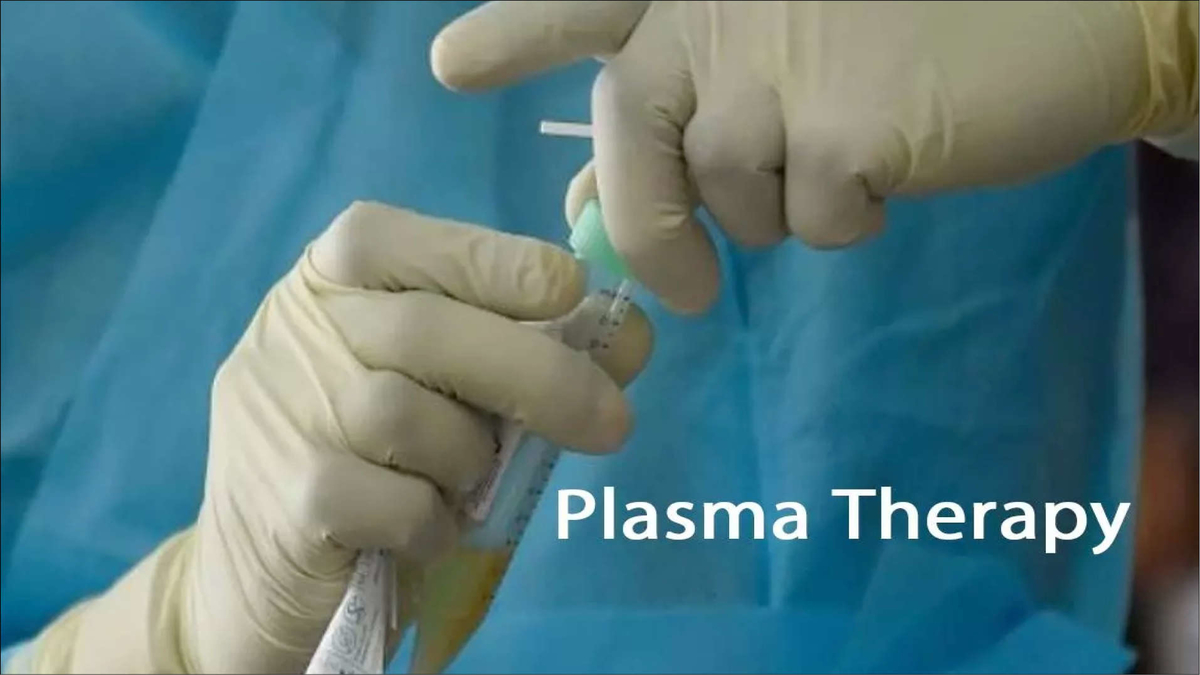
For the past few months, you may have noticed that users on social media have been continuously requesting family members of former covid patients that we need plasma, and many individuals have been frustrated that they have not received plasma treatments in some way. You’ve probably heard many stories about patients who couldn’t live because they didn’t have access to plasma therapy, and there’s been a lot of talk about if the plasma is found, the patient will survive; otherwise, survival is problematic. However, during the night of May 17, the Government of India, which operates with a National Task Force, abruptly intervened. In its recommendation, the National Task Force eliminated plasma treatment from the Clinical Management Guidelines.
As a result, the Indian government no longer recommends that plasma therapy be offered to any hospital or clinic with a covid patient. Many people are perplexed as to why the government abruptly discontinued plasma therapy, which was saving lives. Today in this article, we will know why it was allowed, why it was withdrawn, whether it is appropriate to be removed, and what options are available, so that if any of us has a problem with someone, we will know how to address them and what to avoid.
PLASMA THERAPY: WHAT AND WHY
The antibodies that have been obtained by infected patients will benefit the person who is infected with the corona; this is known as Plasma therapy. The ailment for which you are receiving plasma has been resolved fully. It should also be free of any diseases that could affect him. Antibodies play the most important function in disease transmission because they can protect a person from a new disease that spreads quickly; however, only antibodies unique to this disease may protect a person. Antibody preparation method If there was no vaccination, the only way for antibodies to survive is for us to borrow antibodies from the person whose body have antibodies and save his life.
WHY PLASMA THERAPY WAS APPROVED FOR COVID-19
Plasma treatment was approved for COVID19 because it was used to treat the Spanish flu in 1918, the Swine flu in 2012, and EBOLA in 2014. This treatment was also a ready-to-use way for treating infectious diseases. Aside from that, there was no treatment, though the government was testing this therapy for COVID19 as well.
WHY IS PLASMA THERAPY DROPPED NOW?
Convalescent plasma therapy should be removed from treatment guidelines, according to all members of the ICMR-National Task Force on COVID-19, because it is ineffective and often used inappropriately. The government amended clinical recommendations for COVID-19 treatment on 17 may, removing the off-label use of convalescent plasma after finding it to be ineffective in slowing the progression of severe Covid-19. Last month, the ICMR-National Task Force for COVID-19 met and made the decision. Convalescent plasma should be removed from the guidelines, according to all members of the panel, because it is “ineffective and unsuitable” in many circumstances.
According to PTI, the task committee “updated” the Clinical Guidance for Management of Adult COVID-19 Patients and “deleted convalescent plasma (off label).” Doctors could use plasma therapy on patients with mild symptoms within seven days of beginning of symptoms if a high titre donor plasma was available, according to earlier guidelines.
Some doctors and scientists, including vaccinologist Gagandeep Kang, surgeon Pramesh CS, and others, have written to Principal Scientific Adviser K Vijay Raghavan, urging him to avoid using convalescent plasma for COVID-19 because it is “irrational and non-scientific.”
The letter, which was also addressed to ICMR Director Balram Bhargava and AIIMS Director Randeep Guleria, stated that current plasma therapy guidelines are not based on existing evidence, and that some preliminary evidence suggests a possible link between the emergence of variants with lower susceptibility to neutralising antibodies in immunosuppressed people receiving plasma therapy.
Irrational use of plasma therapy, according to these scientists and clinicians, could contribute to the production of more virulent strains, fueling the pandemic. “This is the result of government-issued recommendations, and we urgently request your intervention to resolve the situation so that COVID-19 patients, their families, professionals, and survivors are not harassed,” the letter stated.
And one of the major reasons to discontinue the plasma therapy is that India has adopted vaccination program which is more effective than plasma therapy and it is long lasting acquired immunity and it is better option as compared to plasma therapy.
PLASMA THERAPY’S COMPLEXITIES
The challenge of getting considerable amounts of plasma from survivors makes this therapy difficult to implement.
Not all recovered patients can offer to donate blood in disorders like COVID-19, where the majority of victims are elderly and have additional medical issues like hypertension, diabetes, and so on.
IS PLASMA THERAPY RELATED WITH NEW VARIENTS?
In a letter to the principal scientific advisory, ICMR president Balram Bhargava, and AIIMS director Randeep Guleria, 18 health professionals stated that when plasma treatment was utilised on an immunosuppressed patient, new variations were produced from the studies. and it was noted in the letter that there was a remote possibility of new variations emerging through plasma therapy. A treatment for which there is no cure for the disease and the possibility of new versions must be discontinued.
CONCLUSION
Medical professionals have proven that plasma treatment is not useful. It is a source of harassment for society, as well as a significant risk, as new varieties are possible. As a result, the Indian government has removed this therapy from COVID19 clinical management guidelines. Patients who require plasma therapy have a far better option in the form of monoclonal antibodies and antibody cocktail