In wake of irregularities being reported continuously at medical stores namely having no sale drug license, non-maintenance of records, medicines stored improperly, no labels on medicines etc. the Food and Drug Authority( FDA) department has come up taking stringent action against the defaulters involved in breach of norms.
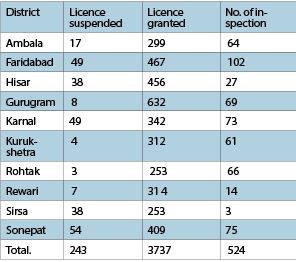
In this series, it came to light that in a period of 7 months, licenses of about 250 medical stores in Haryana have been cancelled. In a bid to ensure the implementation of rules and regulations, FDA is ensuring strict action in different ways against the defaulters: According to the information received by the FDA, action has been taken against 243 medical operators reported involved in noncompliance that led to the cancellation of their licences.
Notably, all 22 districts of the state have been divided into 10 zones while maximum licenses of medical stores have been canceled in Ambala and Karnal. In these, licenses of 98 medical stores, 49 each, have been cancelled, comprising around 40 percent of the total cancelled licences. After this, licenses of 38 medical stores each have been canceled in 76 cases in Sonipat and Hisar zones. Besides, the licenses of 30 medical stores in Sirsa zone, 17 in Ambala, 8 in Gurugram, 7 in Rewari, 4 in Kurukshetra and 3 in Rohtak have been cancelled.
The statistics depicted that as many as 514 manufacturing units (in both categories-Retail and wholesale) have been inspected by State Drug Controller Officers (SDCOs) in different zones and districts in Haryana. Of these, maximum inspection has been done in 102 units in Faridabad district, followed by 75 inspections in manufacturing units in Sonipat zone, 73 and 69 manufacturing units in Gurugram and Karnal have been inspected respectively.
Similarly, a total of 66 manufacturing units have been inspected in Rohtak, 64 in Ambala, 27 in Hisar, 21 in Kurukshetra, 14 in Rewari and 3 in Sirsa. At the same time, it is pertinent to mention that due to an acute shortage of Drug Controller Officers along with administrative staff is continuously adversely affecting the functioning as it continues to result in delayed inspections. The information received has revealed that in the period of 7 months from April to October 2023, FDA has granted 3737 licenses to medical store operators and medicine units in retail and wholesale categories. Among these, a maximum 632 licenses have been granted in Gurugram district units falling in the National Capital Region. Likewise, 467 and 456 medical licenses have been given in adjoining Faridabad and Hisar respectively.
Thus, around 40 percent of the total medical stores licenses have been given in Gurugram, Faridabad and Hisar unit alone. . Apart from these, 409 licenses have been given in Sonipat. Whereas 342 licenses in Karnal, 314 in Rewari, 312 in Kurukshetra, 299 in Ambala, and 506 in Rohtak and Sirsa, 253 each have been granted. During the inspection, it was found that illegal stock was found at many stores which led to lodging FIRs against the defaulters and as many as 37 FIRs have been filed against medical stores that violated the norms. The statistics revealed that a maximum10 FIRs have been lodged in Rohtak district alone while as many as 6 FIRs have been lodged in Jhajjar. Followed by 4 FIRs in Panchkula, 4 in Karnal, 3 in Yamunanagar, 3 in Panipat and 2 in Bhiwani. Apart from these, 4 FIRs have been registered in Ambala, Fatehabad, Hisar, Mewat and Sonipat. 1 each.
Divulging more information pertaining to this, Ashol Meena, the commissioner of FDA said that no one will be allowed to put people’s lives at stake so medical store operators are required to comply with all the rules and regulations. ‘’In a bid to ensure the same, the FDA is leaving no stone unturned to come up with stringent action including lodging FIR’s, cancellation of licences and legal action etc against the defaulters. Through continuous inspection, it is ensured that banned medicines and drugs are not sold at any medical store at higher prices’’,he added.
It is worth noting that action was ensured in wake of the violation of Narcotics Drugs and Psychotropic Substances (NDPS) Act, 1985 . The Food and Drugs Administration (FDA) department jointly conducted the raid with the police. After this, action was taken against those who flouted the rules. During the raid by the department’s police, it was also revealed that many chemists had kept illegal stock of medicines at the medical store. Apart from this, intoxicants were also being sold.