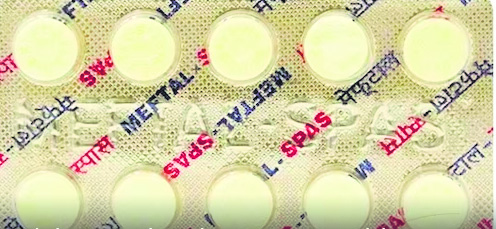
The Indian Pharmacopoeia Commission (IPC) released a drug safety advisory, advising both healthcare providers and patients to observe potential adverse responses linked to the pain reliever Meftal. Typically prescribed for menstrual cramps and rheumatoid arthritis, Meftal contains mefenamic acid and is utilized in managing conditions like mild to moderate pain, fever, inflammation, dental pain, osteoarthritis, and dysmenorrhea.
The commission’s alert, issued on November 30, highlighted a preliminary analysis of adverse drug reactions from the Pharmacovigilance Programme of India (PvPI) database, revealing instances of drug reactions leading to eosinophilia and systemic symptoms (DRESS) syndrome.
“Healthcare professionals, patients/consumers are advised to closely monitor the possibility of the above adverse drug reaction (ADR) associated with the use of the suspected drug,” according to the alert, issued on November 30.
In the event of such reactions, individuals are encouraged to report the matter to the national coordination centre of the PvPI under the commission. This can be done by filing a form on the website www.ipc.gov.in or through the android mobile app ADR PvPI and the PvPI Helpline No. 1800-180-3024.
As an autonomous institution of the Ministry of Health, the IPC establishes standards for all drugs manufactured, sold, and consumed in India.